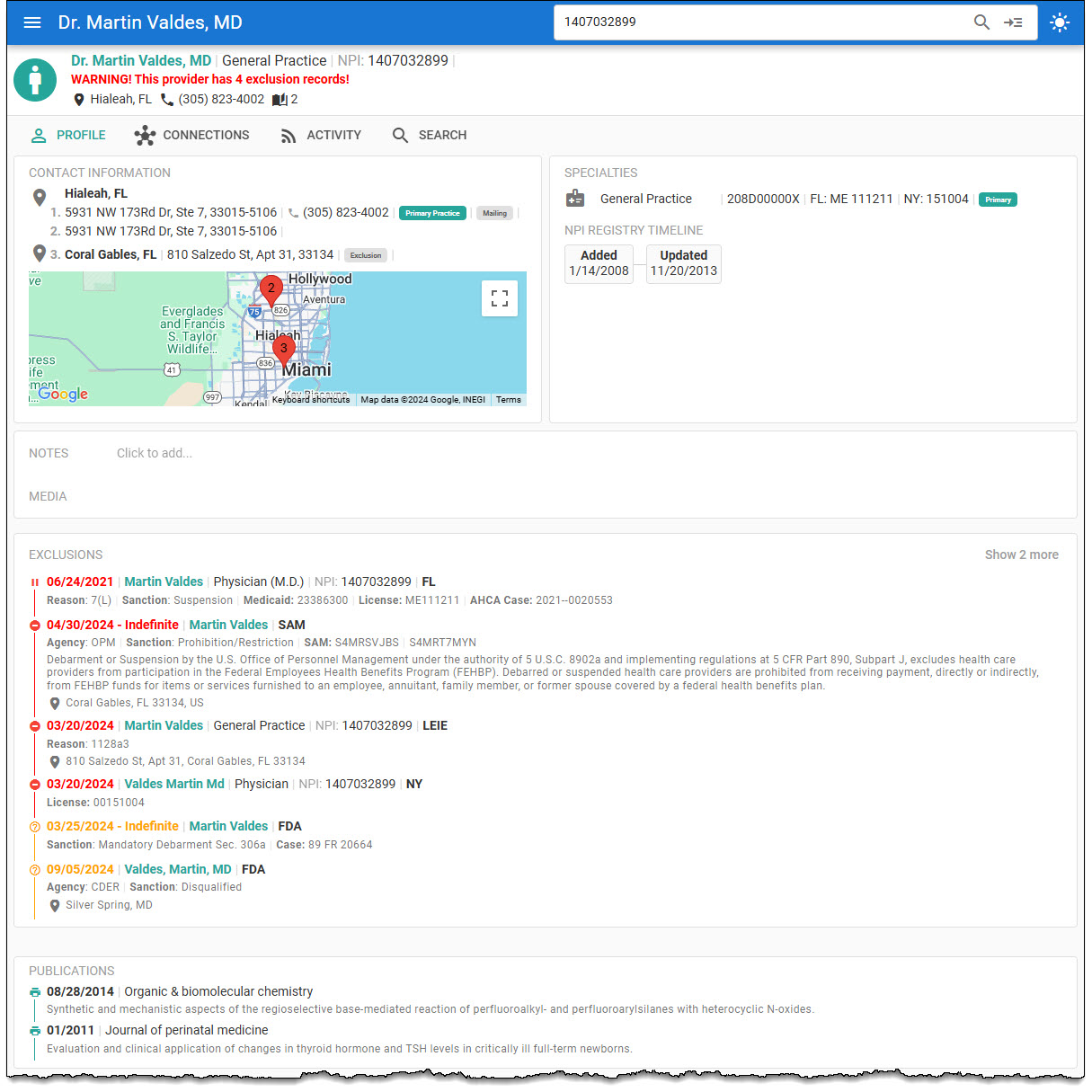
Health Providers DB includes the Food & Drug Administration (FDA) Clinical Investigators—Disqualification Proceedings and FDA Debarment List in the Health Provider’s profile.
Search the Clinical Investigators—Disqualification Proceedings for individuals and companies subject to an administrative, clinical investigator disqualification action per federal regulation 21 CFR Part § 812.119.
Individuals and companies are debarred or sanctioned for abuse, fraud, or integrity issues under sections 306(a) or (b) of the Federal Food, Drug, and Cosmetic Act 21 U.S. Code § 335a — Debarment, temporary denial of approval, and suspension or the Federal Food, Drug, and Cosmetic Act 21 U.S. Code § 335b — Civil penalties as published in the Federal Register and are prohibited from providing services, participating in transactions, or conducting general business with the U.S. Food and Drug Administration.
The FDA Debarment List is updated monthly in the Health Provider DB.
The Clinical Investigators—Disqualification Proceedings are updated monthly in Health Provider DB.