Explore Clinical Trials for Healthcare Providers, Investigators, and Key Opinion Leaders.
HealthProviders DB includes the National Library of Medicine (NLM) ClinicalTrials.gov clinical trials with the Healthcare Provider’s Profile.
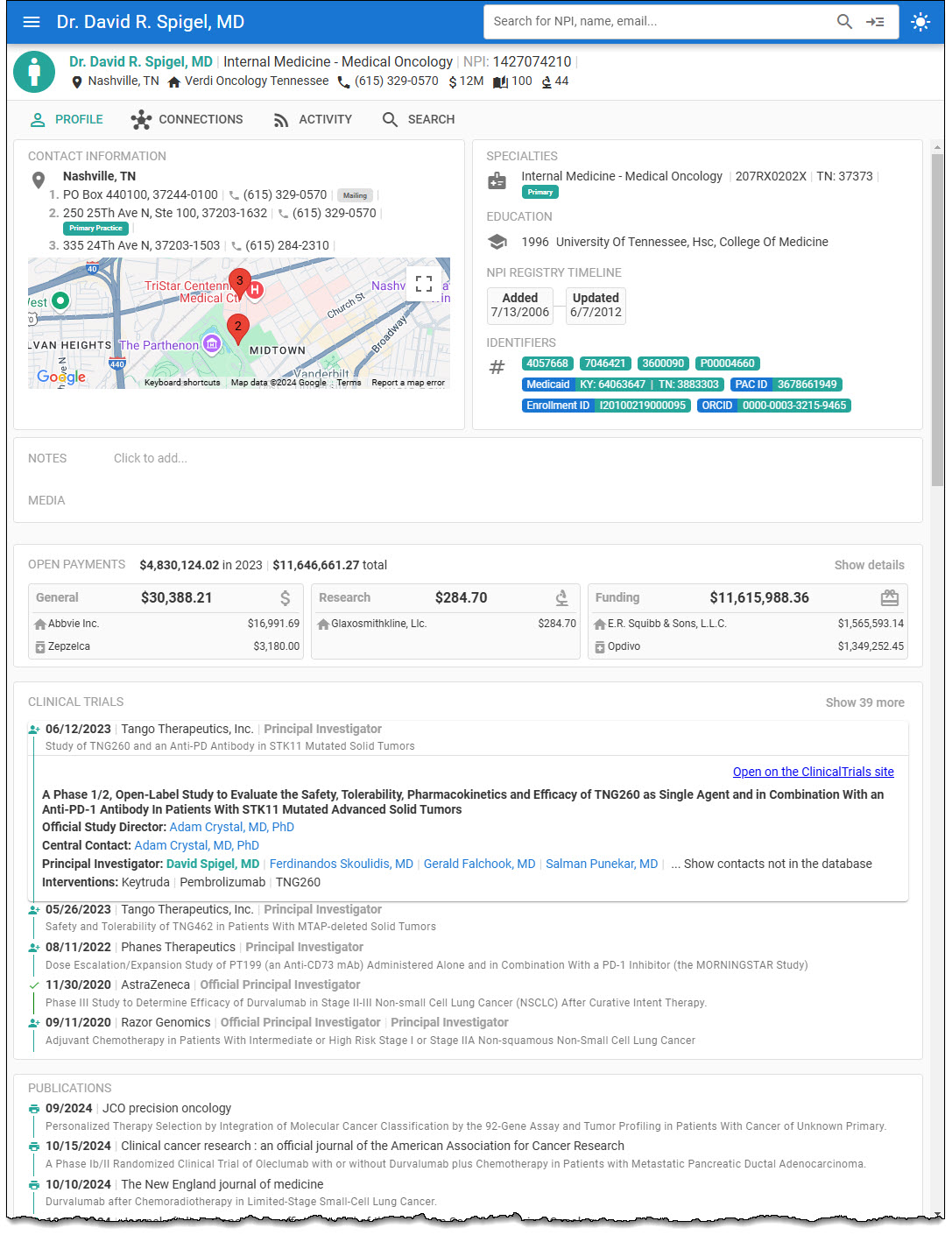
Profiles are identified as the Study Chair, Study Director, Study Principal Investigator, Site Principal Investigator, or Site Sub-Investigator in the health provider’s profile.
The Clinical Trial NCT ID, Official Title, Conditions, Phase, Status, Keywords, and Intervention are included, with a link to the Clinical Trial on ClinicalTrials.gov.
Additionally, you can click on other investigators for the clinical trial to view their profiles.
The ClinicalTrials.gov data is updated daily in the Health Provider DB.
About the NLM ClinicalTrials.gov
ClinicalTrials.gov is a clinical trial database established by the National Institute of Health (NIH) as a central resource to provide current information on clinical trials to members of the public and healthcare providers.
ClinicalTrials.gov results from a federal law requiring the registration of clinical trials. The law aims to enhance public access to information about clinical research, foster public trust in research, and inform future research endeavors.
ClinicalTrials.gov is run by the National Library of Medicine (NLM) at the NIH. It is the largest clinical trials database, encompassing both privately and publicly funded clinical studies conducted worldwide.
- ClinicalTrials.gov relies on sponsors or investigators to submit and update study information.
- ClinicalTrials.gov lists up-to-date information on clinical research studies and their results, adding new studies almost daily.
- ClinicalTrials.gov encompasses studies in all 50 states and more than 200 countries.
- ClinicalTrials.gov supports laws, regulations, and policies requiring sponsors and investigators to share information about clinical trials, including results, publicly.

PubMed is another resource managed by the National Library of Medicine. A trial with an NCT identification number registered in ClinicalTrials.gov can be linked to a journal article with a PubMed identification number (PMID).
A 2013 study analyzing 8907 interventional trials registered in ClinicalTrials.gov found that 23.2% had abstract-linked result articles, and 7.3% had registry-linked articles. 2.7% of trials had both types of links. Most trials are linked to a single result article (76.4%). The study also found that 72.2% of trials had no formally linked result article.
Clinical Trial Data Collected
HealthProviders DB includes the following details from the Clinical Trials database. Links to the Clinical Trial data are provided for reference and research.
| Field Name | Clinical Trials Module |
|---|---|
| NCT ID | protocolSection.identificationModule.nctId |
| Brief Title | Tells you the purpose of the study and how it will work. protocolSection.identificationModule.briefTitle |
| Official Title | The title given to the study by the researchers is often written in scientific language. protocolSection.identificationModule.officialTitle |
| Conditions | The focus of the study is usually a disease, illness, or condition, but it may also be another health-related issue. protocolSection.conditionsModule.conditions |
| Phases | The stage of the study is based on definitions from the U.S. FDA. protocolSection.designModule.phases |
| Status | protocolSection.statusModule.overallStatus |
| Official Name | protocolSection.contactsLocationsModule.overallOfficials.name |
| Official Affiliation | protocolSection.contactsLocationsModule.overallOfficials.affiliation |
| Official Name | protocolSection.contactsLocationsModule.overallOfficials.role Study Chair Study Director Study Principal Investigator |
| Investigator Name | protocolSection.sponsorCollaboratorsModule.responsibleParty.investigatorFullNam |
| Investigator Affiliation | protocolSection.sponsorCollaboratorsModule.responsibleParty.investigatorAffiliation |
| Investigator Role | protocolSection.sponsorCollaboratorsModule.responsibleParty.role Site Principal Investigator Site Sub-Investigator |
| Keywords | Helps potential participants and other interested parties find the study. |
| Interventions | The treatment, device, or procedure that’s being studied. |

